YUNCO Holds 2024 University Student Summer Social Practice Reception
On July 10, another batch of six students from the College of Optics and Electronics Information at Huazhong University of Science and Technology visited. The Advanced Manufacturing System Innovation Service Platform for Medical Devices, which has already received over 12 outstanding talents from universities such as the School of Mechanical Science and Engineering, the College of Optics and Electronics Information at Huazhong University of Science and Technology, and the College of Optoelectronic Engineering at Soochow University in the field of medical-engineering interdisciplinary industry innovation practice.


Since 2023, YUNCO Medical has been actively expanding its training for engineering talents, laboratory open day activities, and high-end device product realization services such as "new materials-new processes-new applications" to further strengthen the construction of the advanced manufacturing system innovation service platform for medical devices. We have actively strengthened project cooperation with institutions such as 301 Hospital, Fuwai Hospital, Gulou Hospital of Nanjing University, Tsinghua University, Peking University, Shanghai Jiao Tong University, Zhejiang University, Fudan University, University of Shanghai for Science and Technology, Huazhong University of Science and Technology, Institute of Physics of the Chinese Academy of Sciences, and Institute of Chemistry of the Chinese Academy of Sciences. We have established provincial graduate workstations, graduate practice bases, municipal-level high-end medical device manufacturing systems and process engineering technology research centers, and carried out school-enterprise cooperation scientific research projects with universities, achieving fruitful results. We are equipped to carry out industrialization conditions, and the medical-engineering interdisciplinary industry innovation practice is growing rapidly and effectively.
YUNCO Medical Device Advanced Manufacturing System Innovation Service Platform

The Advanced Manufacturing System Innovation Service Platform for Medical Devices relies on YUNCO's ten years of strong technical research and application market expansion capabilities in the three major fields of interventional medical devices, minimally invasive surgical instruments, and endoscopic snake bones.
Core Product Line 1: Laser Micromachining and Supporting Systems for Interventional Medical Devices
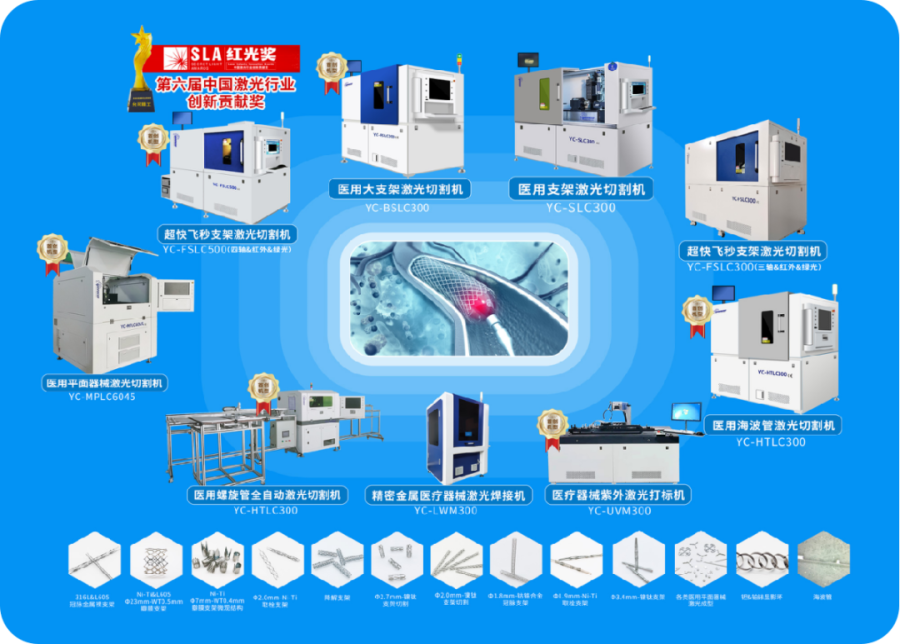
The first domestic team to develop medical stent laser cutting systems, the first domestic drug-coated stent laser cutting machine, interventional valve stent laser cutting machines, laser cutting and engraving machines for hypodermic tubes & automatic loading and unloading systems, ultra-fast femtosecond laser processing centers, and micro blade grinding and polishing systems. We provide laser micromachining and innovative service system solutions for interventional medical devices such as metal bare stents, drug-coated stents, tissue-engineered stents, biodegradable stents, flexible hypodermic tubes, catheters, filters, interventional valve stents, marker rings, stone extraction baskets, balloons, and micro blades.
Core Product Line 2: Laser Micromachining and Supporting Systems for Minimally Invasive Surgical Instruments

In 2014, the industry’s first five-axis laser micromachining system for surgical instruments was launched. In 2015, the first domestic medical blade grinding and polishing system was launched. Subsequently, laser micromachining and innovative service system solutions for minimally invasive surgical instruments such as ultrasonic scalpels, endoscopes, staplers, rigid endoscopes, puncture needles, RF ablation needles, cosmetic needles, indwelling needles, egg retrieval needles, orthopedic connection pieces, soft knives, and planer knives for sports medicine instruments were introduced.
Core Product Line 3: Laser Micromachining and Supporting Systems for Endoscopic Snake Bones
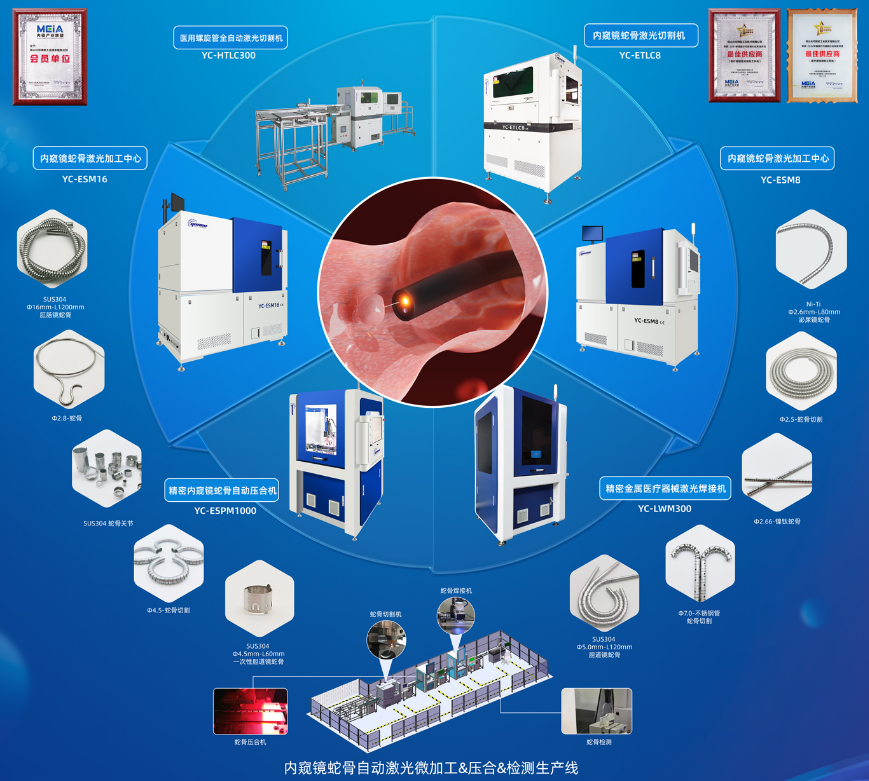
In 2015, the first specialized machine for laser cutting endoscopic snake bones was launched. In 2016, the first endoscopic snake bone laser processing center was launched. In 2017, the endoscopic metal instrument laser welding system was launched. In 2018, the first automatic pressing system for endoscopic snake bones was launched. In 2021, the first automatic production line for endoscopic snake bones was launched. These solutions have been rated as the best supplier of laser micromachining systems for medical devices by the China Endoscopy Alliance in 2021 (first time) and 2022.
Innovative Product Line 1: Series of Key Functional Modules
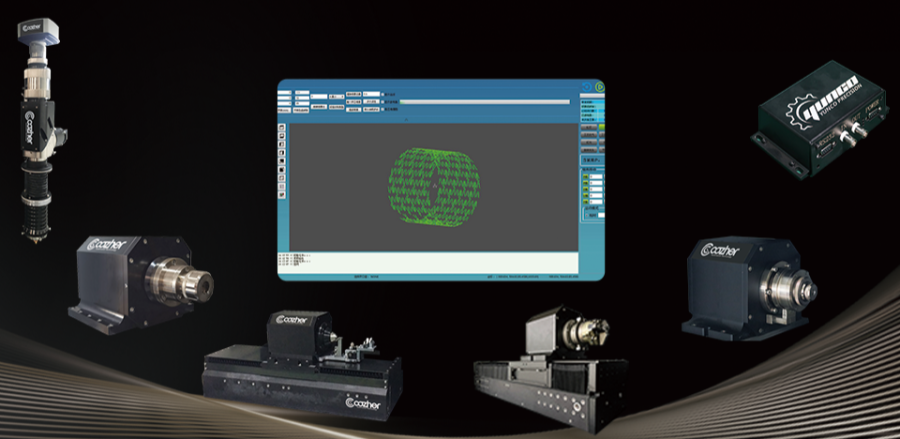
We offer fine laser cutting heads, precision rotating shafts, precision motion platforms, precision linear shafts, laser signal controllers, precision clamping fixtures, and laser micromachining software systems. Our modular, forward-design concept builds world-class manufacturing system design and manufacturing iteration capabilities.
Innovative Product Line 2: Precision Intelligent Manufacturing System Solutions for Medical Devices

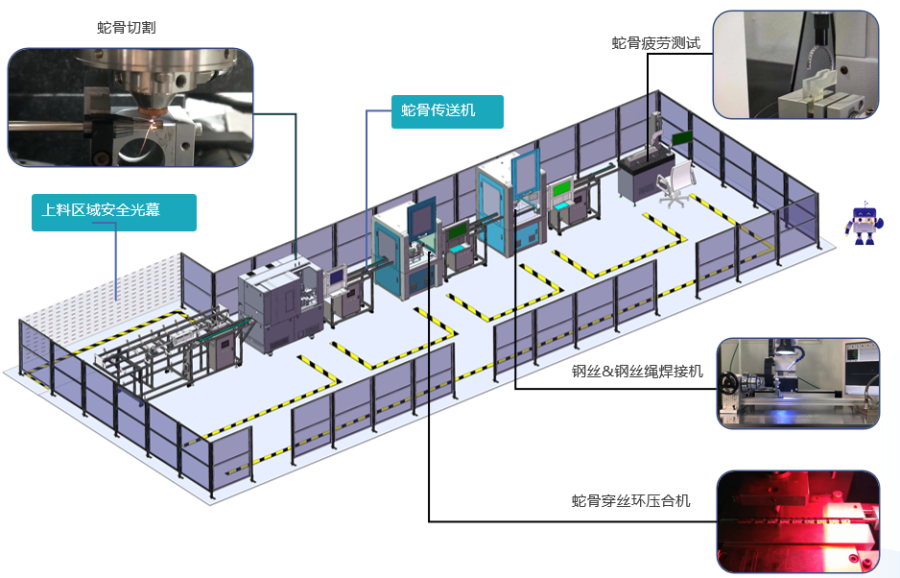
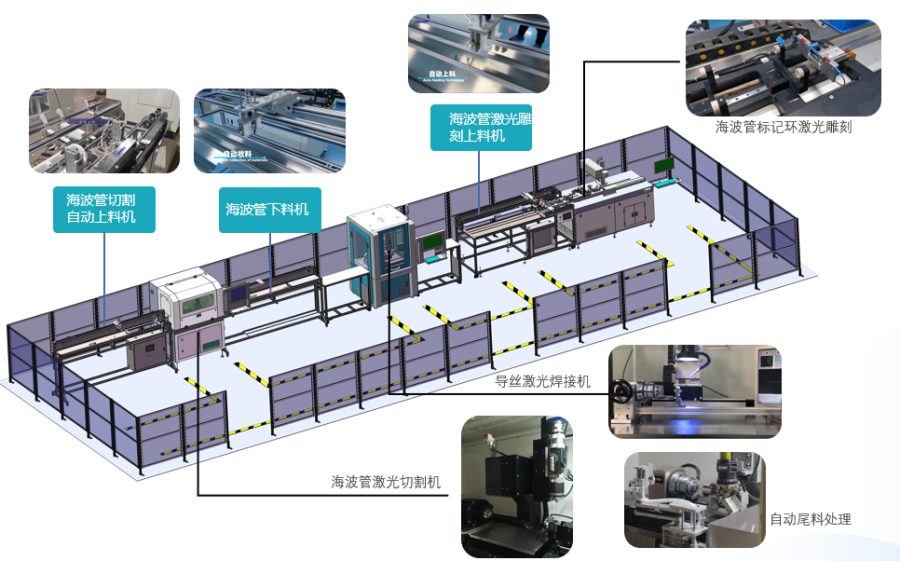
We provide innovative services for replacing old with new systems, automatic production lines for endoscopic snake bones & insertion tubes, automatic production lines for hypodermic tubes & catheters & needles, and precision intelligent manufacturing system solutions under the demand for medical device consumables and centralized procurement—automation production.
Innovative Product Line 3: CDMO Innovation Services for Medical Devices

We strengthen medical-engineering collaboration and are dedicated to providing a closed-loop research and development platform from new processes and engineering technology innovation service systems to medical device pre-research design, engineering verification, and clinical testing. This solidifies the foundation for innovative design of medical devices. We break down collaboration barriers between medical experts, medical device R&D teams, and medical device engineering production teams, shorten the R&D cycle of medical devices, and reduce R&D costs.
One-stop Innovative Service Capabilities for Medical Device CDMO
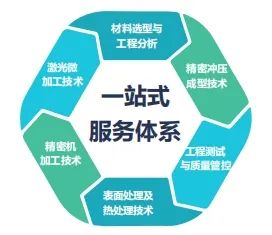
We provide precision manufacturing, laser engineering, innovative services, and project conversion:
- **Material Selection and Engineering Analysis:** Analyzing the machinability of materials to determine if they meet the final performance requirements of the device, establishing a material comparison analysis database to support the determination of material requirements for device design.
- **Engineering Testing and Quality Control:** Engineering testing of machined parts or assembled components, establishing a quality control database for parts processing and assembly to provide basic data support for mass production or production technology transfer.
- **Precision Machining Technology:** Supporting precision machining centers, Swiss-type lathes, internal and external grinding machines, precision turning and milling composite processing machines, CNC equipment with laser processing capabilities, providing sub-micron level processing capabilities.
- **Precision Stamping and Forming Technology:** Providing precision stamping and forming processes such as expansion and contraction for precision tube and planar instruments.
- **Laser Micromachining Technology:** Providing micron-level precision processing capabilities such as laser cutting, welding, marking, engraving, etching, surface texturing, and microporous processing for tube or planar instruments, offering customized services for components, specialized machines, and automated systems.
- **Surface Treatment and Heat Treatment Technology:** Using physical and chemical processes to provide ultrasonic cleaning, acid cleaning, polishing, sandblasting, passivation, heat setting, and other surface and heat treatment capabilities for machined parts.


